Home » Scientific%20research%20progress

Abstract
Quantification of nanoparticle-molecule interaction at a single-molecule level remains a daunting challenge, mainly due to ultra-weak emission from single molecules and the perturbation of the local environment. Here we report the rational design of an intraparticle-surface energy transfer (i-SET) process, analogous to high doping concentration-induced surface quenching effects, to realize single-molecule sensing by nanoparticle probes. This design, based on a Tb3+-activator-rich core-shell upconversion nanoparticle, enables a much-improved spectral response to fluorescent molecules at single-molecule levels through enhanced non-radiative energy transfer with a rate over an order of magnitude faster than conventional counterparts. We demonstrate a quantitative analysis of spectral changes of one to four fluorophores tethered on a single nanoparticle through i-SET spectroscopy. Our results provide opportunities to identify photoreaction kinetics at single-molecule levels and provide direct information for understanding behaviors of individual molecules with unprecedented sensitivity.
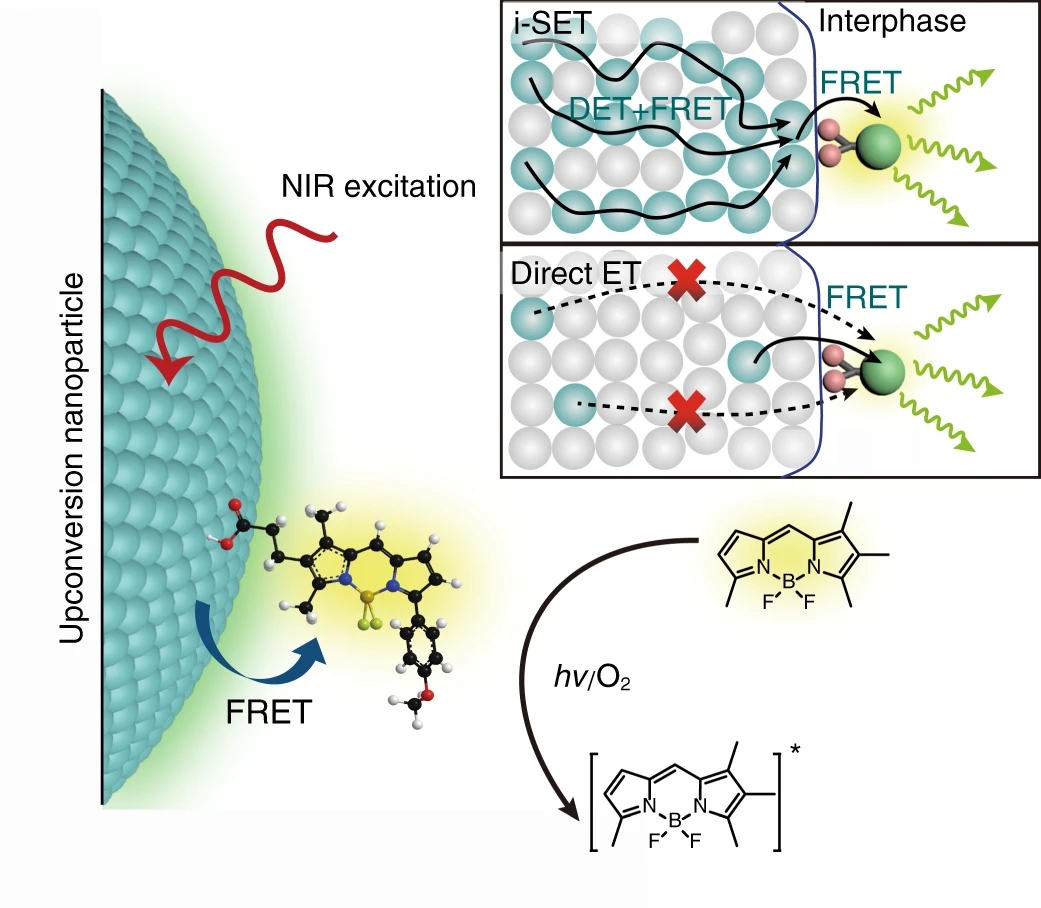
Fig. 1: Schematic of single upconversion nanoparticle-based molecular sensing.The sensing of individual molecules is realized by probing the FRET signal of nanoparticle-to-molecule energy transfer. The i-SET path shows energy transfer from nanoparticles with a high content of activators (represented as cyan spheres) in which excitation energy can fast migrate within the particles by either Dexter energy transfer (DET) or FRET and lead to promoted sensitization of adhesive molecules through intraparticle-to-surface FRET. The direct ET path indicates energy transfer from conventional upconversion nanoparticles having low doping concentration of activators in which activators buried inside the nanoparticle can hardly transfer their energy to the molecular acceptors due to the longer distance of separation.
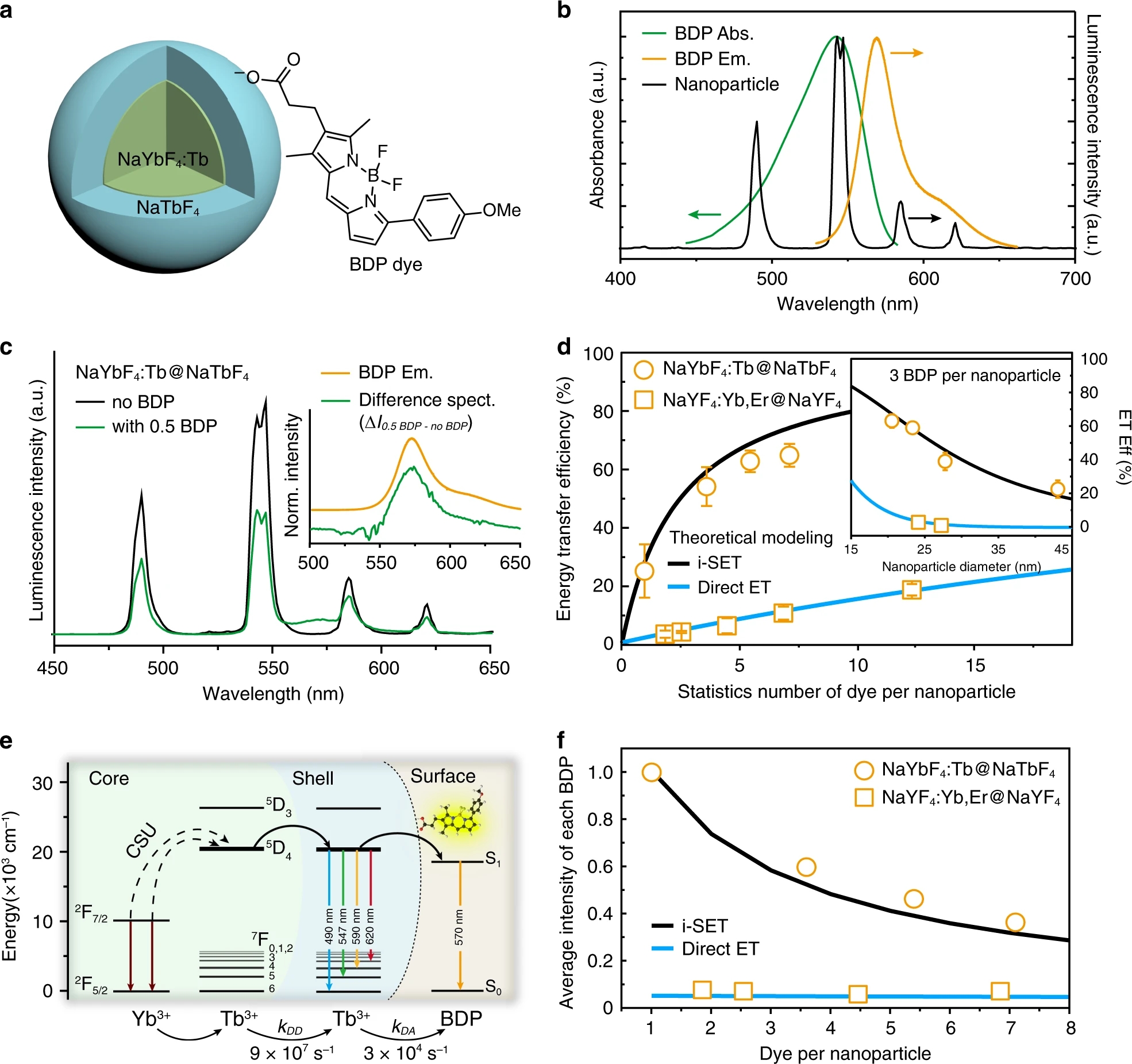
Fig. 2: Experimental and theoretical investigations of energy transfer between upconversion nanoparticles and organic dyes.a Schematic illustration of a NaYbF4:Tb(40 mol%)@NaTbF4 core-shell upconversion nanoparticle coupled with a carboxylic acid-functionalized borondipyrromethene dye (BDP dye) in our study. b Normalized UV�Cvis absorption (Abs.) and emission (Em.) spectra of BDP dyes, and upconversion luminescence (PL) spectrum of NaYbF4:Tb(40 mol%)@NaTbF4 nanoparticles. c Upconversion luminescence spectra of NaYbF4:Tb(40 mol%)@NaTbF4 nanoparticles conjugating with and without BDP conjugation. The inserted diagram shows shows a comparison between the direct emission spectrum of free BDP molecules at 365 nm excitation and the sensitized emission spectrum of coupled BDP obtained by calculating the spectral differences between emission spectra of the nanoparticles with and without 0.5 BDP conjugation. d Theoretical plots and experimental data of energy transfer efficiencies for NaYbF4:Tb(40 mol%)@NaTbF4 and NaYF4:Yb,Er(18,2 mol%)@NaYF4 core�Cshell nanoparticles with the same diameter of 24 nm as a function of average BDP molecules per nanoparticle. Inset is energy transfer (ET) efficiency plotted as a function of particle size for nanoparticles decorated with an average of three BDP per particle. The error bars represent one standard deviation of three parallel experiments. e Proposed energy transfer mechanism describing the i-SET from NaYbF4:40%Tb@NaTbF4 core�Cshell nanoparticles to BDP dyes. f Simulated and experimental average luminescence intensity of single BDP molecule as a function of BDP coverage on individual nanoparticles.
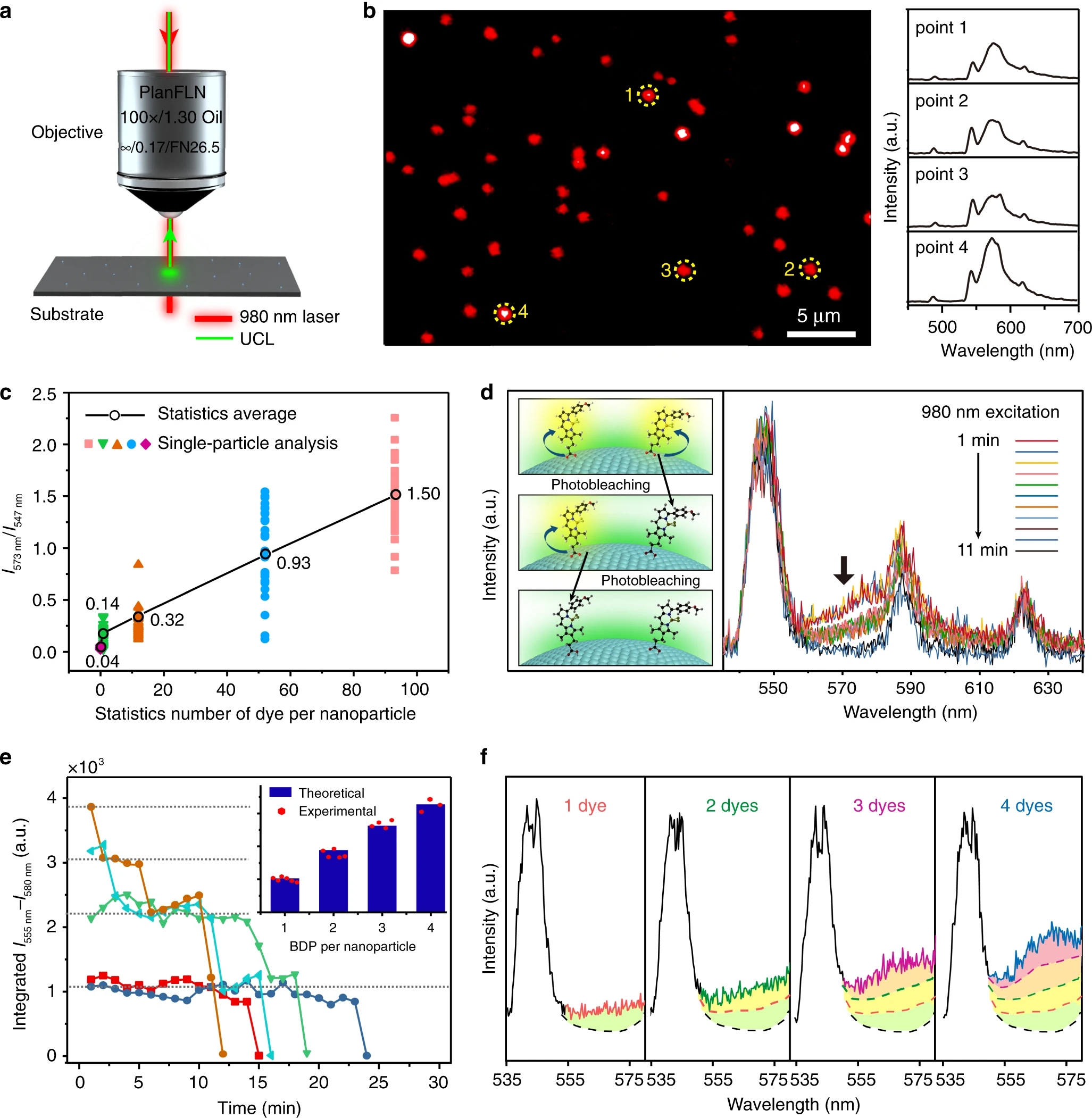
Fig. 3: Single-particle characterization of BDP dye-modified upconversion nanoparticles through i-SET spectroscopy.a Schematic of the experimental design for detecting single-upconversion luminescence (UCL) from of dye-decorated upconversion nanoparticles using a confocal microscope imaging system. b A typical confocal microscope image of NaYbF4:Tb(40 mol%)@NaTbF4 nanoparticles loaded with an average of ~92 BDP molecules per nanoparticle (left panel), and corresponding single-particle upconversion luminescence spectra (right panel) recorded at the points marked with yellow dashed circles. c Plots of sensitized BDP emission to Tb3+ emission intensity ratios measured at single-particle level from a series batches of BDP-decorated NaYbF4:Tb(40 mol%)@NaTbF4 nanoparticles with different average coverage ratios of dye molecules. Each of the colour dots represent an individual spectral measurement from a randomly picked nanoparticle, and the black circles represent the average ratios of individual measurements from the same sample glass substrates. d Time-dependent upconversion luminescence spectra of a typical BDP-decorated upconversion nanoparticle showing discrete two-steps photobleaching of sensitized BDP emission at around 573 nm under 980 nm irradiation. The inserted scheme illustrates the step-wise single-molecule photobleaching of two BDP molecules at the surface of a nanoparticle. e Time-dependent integrated emission intensity changes of BDP recorded from several individual single-particle measurements. The inset shows a comparison between the theoretically calculated dye emission intensity ratios and the experimental intensity ratios extracted from the single-particle measurements. f Single-particle upconversion luminescence spectra of individual NaYbF4:Tb(40 mol%)@NaTbF4 nanoparticles conjugated with 1, 2, 3, and 4 active dye molecules, respectively. The nanoparticle-sensitized BDP emissions are highlighted in colours.
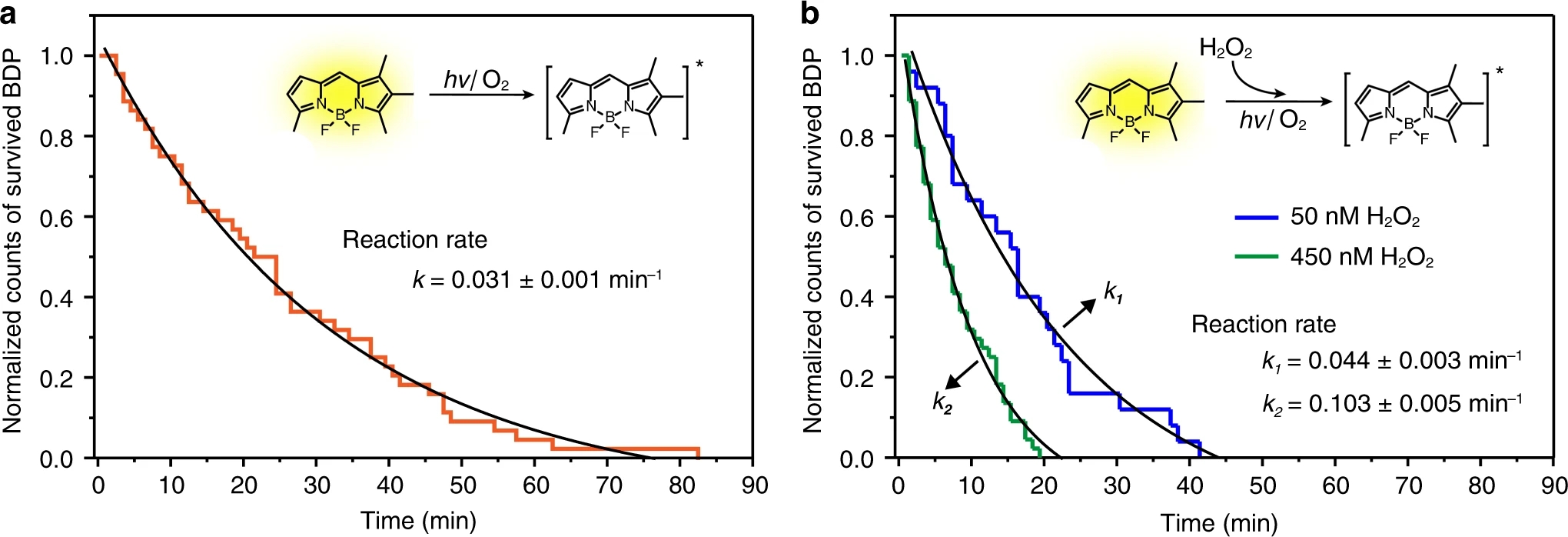
Fig. 4: Kinetic investigation of single-molecule photoreactions.a Normalized counting of survived BDP molecule collected from 45 individual single-molecule measurements as a function of irradiation time. It reveals photobleaching kinetics of the single-molecule reaction of BDP at the surface of NaYbF4:Tb(40 mol%)@NaTbF4 upconversion nanoparticles. b Plots of normalized counting of survived BDP molecule versus irradiation time indicating the single-molecule reaction of BDP accelerates after exposure to a different amount of H2O2.
Discussion
In conclusion, we report the generation of efficient single-molecule upconversion nanoprobes by applying a high-activator-content-promoted i-SET approach. We demonstrate that factors known to be harmful to luminescence efficiency in accelerating surface quenching may be beneficial to energy donation from the nanoparticles to their surface-modified molecular acceptors. Our results establish a methodology to enable precise control over the energy transfer pathways between luminescence nanoparticles and various types of functional targets. The significant enhanced nanoparticle-to-molecule interaction enables us to quantitatively probe single-molecule behaviors on a nanoparticle with ultimate precision. These nanoprobes should be potentially applicable to the evaluation of the effects of different surface modifications on the heterogeneity of organic-nanoparticle hybrids. They may also be useful for understanding photochemical properties of individual molecules on nanomaterial surfaces. Our findings could open new avenues for the design of high-performance single-particle upconversion spectroscopy, particularly suitable for applications in photosensitization, photocatalysis, and single-molecule characterization.
Single-molecule photoreaction quantitation through intraparticle-surface energy transfer (i-SET) spectroscopy. Jian Zhou, Changyu Li, Denghao Li, Xiaofeng Liu, Zhao Mu, Weibo Gao, Jianrong Qiu, Renren Deng Nat. Commun., 2020, 11, 4297, DOI: 10.1038/s41467-020-18223-z
Website��https://www.nature.com/articles/s41467-020-18223-z

