Home » Scientific research progress

Highlights
-
Universal design principle of ionic conductors toward durable Li anode
-
Local environment and dynamics of ions in MOF-based semi-solid interphase
-
High-energy-density Li-metal batteries demonstrated under realistic conditions
Progress and Potential
Summary
Introduction
Toward the manufacture of high-energy-density batteries, metallic lithium (Li) is considered as the ultimate choice for anodes in view of its highest capacity (3,860 mAh g−1) and lowest potential (−3.04 V versus standard hydrogen electrode). Early attempts to utilize Li-metal anodes (LMAs) in the 1980s ended up with safety concerns associated with internal short-circuit caused by growth of Li dendrites. Rooting from the electrochemical process, dendrite growth is intrinsically associated with the transport and reaction of Li. Typical solid-electrolyte interphase (SEI) layers formed on the LMA are heterogeneous, consisting of inorganic and organic components with distinct Li-ion conductivities and mechanical properties.
During a charging process (Li deposition), Li ions (Li+) are transported through electrolyte and SEI layer and reduced to metallic Li. The inhomogeneity of the SEI layer, together with convection of Li+ ions in electrolyte, results in the formation of Li nuclei. Meanwhile, faster diffusivity of anions than cations (Li+) in electrolyte (manifested by a low Li-ion transference number, tLi+), along with the continuous consumption of Li+ ions, creates an electrolyte concentration gradient in the vicinity of LMA. Such a concentration gradient provides the protruded sites with a higher Li+ flux, promoting their growth into dendrites through a self-enhanced growth mechanism. In addition, preferable deposition of Li on newly formed sites with a thinner SEI layer (higher Li+ conductivity) can also result in dendrite growth through a root-growth mechanism. Regulating the transport and reaction of Li+ ions in this context is essential to depress dendrite growth and improve the performance of LMA.
To regulate the transport process, we extensively explored electrolyte additives and artificial SEI layers. The use of additives such as fluoroethylene carbonate (FEC) and other fluorinated compounds promoted the formation of more homogeneous SEI layers, helping to improve the performance of LMAs. However, the low modulus of as-formed SEI layers generally is insufficient to withstand the mechanical deformation induced by dendrite growth. In the aspect of artificial SEI layers, inorganic Li+ conductors such as Li3N and LLZO with high Li+ conductivity (��Li+) and high tLi+ were examined; their brittle nature, however, makes the integration of batteries difficult. Polymeric Li+ conductors, on the other hand, could form conformal coatings on Li metal with better integrality, whereas the current single-ion polymeric conductors still exhibit mediocre ionic conductivity. Separators coated with ceramic-electrolyte particles, as well as glass-fiber layers placed between LMA and separator, were also explored to redistribute and homogenize the Li+ flux, leading to LMA with improved performance. To this end, an efficient strategy that can effectively regulate the ionic transport for practical LMAs is still absent. In fact, due to the poorly controlled reaction-transport process, whisker-like rather than dense Li is commonly formed, which is still responsible for the low Coulombic efficiency (CE) and fast degradation of the LMA.
To better regulate the transport and reaction process, an effective artificial SEI layer should possess both high ��Li+ and tLi+ as well as electrochemical stability and ease of integration. We adopt a transport model to predict how the ionic transport properties of interphases/electrolytes affect the Li-deposition morphology. Under a certain current density, the safe capacity that does not trigger diffusion-controlled Li-dendrite formation is directly correlated with tLi+ and diffusion coefficient D (which is related to ��Li+) of a given electrolyte. Artificial SEI layers that render high tLi+ but do not much slow down the ionic transport would enable high cycling capacity. We envision that such artificial SEI layers could be made on the basis of metal-organic frameworks (MOFs), a class of nanoporous materials with designable structure and composition. As a proof of concept, nanoparticles of UiO-66, an MOF made from ZrOx clusters and 1,4-benzenedicarboxylic acid (H2BDC), can be readily deposited on Li foil to form artificial SEI layers that contain nanoporous channels and high density of Lewis-acidic sites. UiO-66 is chosen due to its high (electro-)chemical stability, ease of synthesis, and relatively low cost. During battery assembly, as-added liquid electrolyte is filled within the nanoporous channels, which allows the anions (e.g., PF6−) to complex with the Lewis-acidic sites and the solvated cations (Li+) to effectively translocate within the pore channels with both high ��Li+ and tLi+.
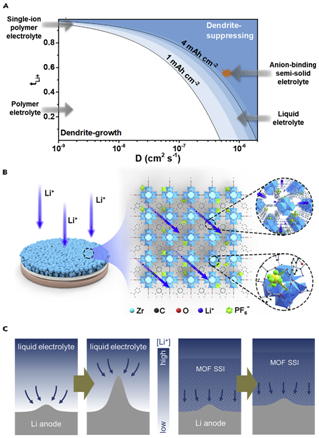
Figure 1Schematic of MOF-Based Semi-Solid Interphase for Stable Li Metal Anode
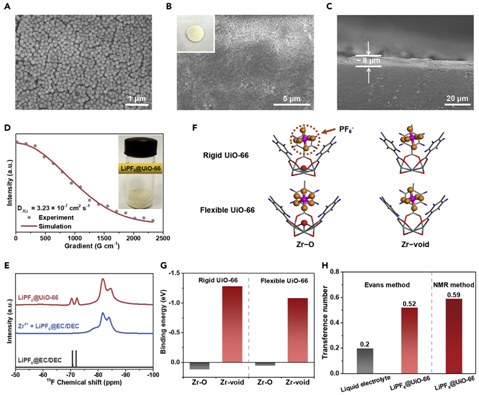
Figure 2Characterizations of MOF-Based SSI on LMA
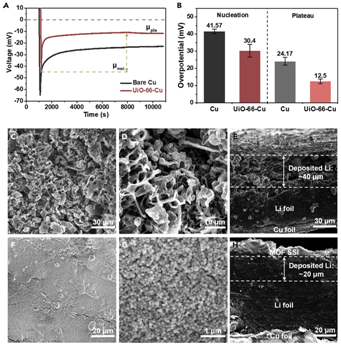
Figure 3Li Deposition with MOF-Based SSI

Figure 4Electrochemical Performance of LMA with MOF-Based SSI
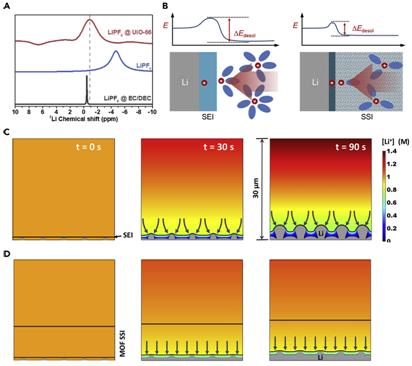
Figure 5Desolvation Process and Calculations of the Ion Concentration
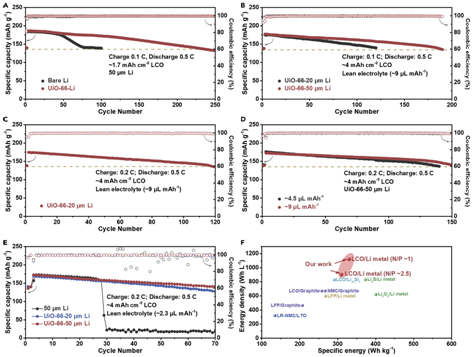
Figure 6Electrochemical Performance of Prototype LMBs
Haobin Wu��https://person.zju.edu.cn/hbwu
Title��Ion-Transport-Rectifying Layer Enables Li-Metal Batteries with High Energy Density
Website�� https://www.cell.com/matter/fulltext/S2590-2385(20)30438-0
DOI��https://doi.org/10.1016/j.matt.2020.08.011

